The Food and Agriculture Organization of the United Nations and the World Health Organization defined probiotics in 2001 as “Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”
Incorporating live microorganisms in food, mainly lactic acid-producing bacteria, has a rich historical tradition of enhancing human health. Ancient civilizations like the Greeks and Romans used fermented dairy products to maintain health. In 76 BC, a Roman historian recommended fermented milk products for treating gastroenteritis. However, the scientific study of microorganisms in fermented foods and their health effects is a relatively recent development.
The journey of probiotics began over a century ago when Henry Tessler of the Pasteur Institute in Paris discovered Bifidobacterium in the intestines of breastfed infants in 1899, noting its benefits for diarrhea. Russian scientist Elie Metchnikoff, a Nobel laureate, proposed 1907 that beneficial microorganisms could replace harmful gut flora. In the early 1930s, Shirota demonstrated that intestinal bacteria could survive the digestive process and developed a fermented milk product containing Lactobacillus acidophilus Shirota, known today as Yakult. In 1965, Lilly and Stillwell coined the term “Probiotic” to describe substances that promote the growth of other microorganisms. Since then, the definition of probiotics has expanded to include various health benefits for the host.
Today, there is a vast commercial market for probiotic nutraceuticals. These products are readily available to consumers without stringent regulatory approvals, leading to a proliferation of products with various claims about gut health and overall well-being.
In recent years, integrating probiotics into antibiotic therapy has garnered significant attention in the medical community. This combination seeks to mitigate the adverse effects of antibiotics while enhancing patient outcomes. The trend of incorporating probiotics into antibiotic therapy began to gain significant attention post-2010. This was driven by increasing awareness of the adverse effects of antibiotics on the gut microbiota, particularly the risk of antibiotic-associated diarrhea (AAD) and other gastrointestinal disturbances. While research has demonstrated the potential benefits of probiotics in maintaining gut health and mitigating these side effects, further research is needed to understand this approach's implications fully.
Guideline and Recommendations
Professional organizations and healthcare providers have developed several guidelines and recommendations regarding using probiotics in conjunction with antibiotics. Listed below are some critical points from notable sources:
- World Gastroenterology Organisation (WGO) Global Guidelines (2023): Recommends using specific probiotic strains to prevent antibiotic-associated diarrhea (AAD) and Clostridium difficile infections (CDI). Strains like Lactobacillus rhamnosus GG and Saccharomyces boulardii are highlighted for efficacy in children and healthy adults. Lactobacillus casei DN-114 001 has been reported effective in hospitalized adult patients in preventing antibiotic-associated diarrhea and C. difficile diarrhea.
- American Gastroenterological Association (AGA) Clinical Practice Guidelines (2020): These guidelines suggest that certain probiotics can prevent difficile infections and AAD in specific patient populations. The AGA recognizes that while some evidence supports the use of probiotics, further research is needed for more robust recommendations.
- European Society for Clinical Nutrition and Metabolism (ESPEN) Guidelines: The guidelines recommend using probiotics in patients receiving antibiotics to reduce the risk of AAD. They emphasize the importance of strain-specific efficacy, with particular strains showing better outcomes in clinical trials.
Probiotic Strains preferred as an adjunct to Antibiotic Therapy:
In antibiotic therapy, several specific probiotic strains are commonly added to mitigate the adverse effects on gut microbiota and to enhance patient outcomes. These strains have been chosen based on their proven efficacy in restoring gut health, preventing antibiotic-associated diarrhea, and supporting overall digestive health. Some of the critical probiotic strains include:
- Lactobacillus rhamnosus GG (LGG): This strain is one of the most extensively studied probiotics and has been shown to effectively reduce the risk of antibiotic-associated diarrhoea and Clostridium difficile infections
- Saccharomyces boulardii: This probiotic yeast is particularly effective in preventing and treating antibiotic-associated diarrhea. It helps to restore gut flora balance and supports gastrointestinal health
- Bifidobacterium lactis: This strain is known for its ability to improve gut transit time and stool consistency, making it beneficial in managing constipation and other digestive issues that may arise during antibiotic
- Lactobacillus acidophilus: This strain helps maintain a healthy balance of gut bacteria, reduces gastrointestinal symptoms, and supports overall digestive health during antibiotic therapy.
- Bifidobacterium longum: Known for its role in supporting immune function and gut health, this strain is often included in probiotic supplements to counteract the adverse effects of antibiotics on the gut microbiome
These strains are chosen for their robust clinical evidence supporting their effectiveness in conjunction with antibiotics, making them integral components of probiotic formulations used in antibiotic therapy.
Understanding the probiotic-antibiotic synergy
While effective in eliminating harmful bacteria, antibiotics often disrupt the balance of gut microbiota, leading to side effects such as diarrhea, yeast infections, and even long-term health issues. Probiotics, which are live beneficial bacteria, can help restore this balance by replenishing the gut with healthy microbes.
Research indicates that probiotics can:
- Studies have shown that certain strains of probiotics have a significant potential to reduce the incidence of Antibiotic-Associated Diarrhea (AAD), particularly in children and elderly patients. This promising research offers hope for a future where the adverse effects of antibiotics can be effectively mitigated.
- Enhance Gut Microbiota Diversity: Probiotics help maintain a diverse and resilient gut microbiome, which is crucial for overall health and immune function.
- Boost Immune Response: Probiotics can modulate the immune system, potentially reducing the need for antibiotics and improving recovery rates.
Market Potential for Probiotic Assessment
The growing acceptance of probiotics as an adjunct to antibiotic therapy presents substantial opportunities for businesses in the probiotic assessment sector. Here’s how this market can be tapped:
- Clinical Research
- Strain-Specific Studies: Conducting a robust literature search and locating sufficient clinical trials can help identify the most effective probiotic strains for specific antibiotics and infections. This can help establish evidence-based guidelines for probiotic use.
- Safety and Efficacy Profiling: Assessing the safety profiles of probiotics, particularly in vulnerable populations such as infants, pregnant women, and the elderly, is essential to gaining regulatory approval and consumer trust.
- Product Development and Customization
- Targeted Probiotic Formulations: Developing probiotic supplements synergizing with commonly prescribed antibiotics can enhance therapeutic outcomes and minimize side effects.
- Personalized Probiotics: Utilizing advances in microbiome sequencing and AI, businesses can create personalized probiotic regimens based on an individual’s unique microbiota composition and health status.
- Consumer Education and Awareness
- Informing healthcare providers and consumers about the benefits and proper use of probiotics in conjunction with antibiotics is crucial in driving market demand and acceptance. By educating others, we can all help promote the responsible use of probiotics.
- Transparent Labelling: Clear and accurate labeling of probiotic products, including strain information and clinical evidence, can build consumer confidence and differentiate products in a crowded market.
- Regulatory Compliance and Quality Assurance
- Adhering to Standards: Ensuring compliance with regulatory standards for probiotic manufacturing and labeling is crucial for market entry and sustainability.
- Offering third-party services for probiotic potency and purity compliance can help businesses establish credibility and trust with consumers and healthcare professionals.
How can Evalueserve IP and R&D help you?
Evalueserve boasts extensive experience in preparing and managing regulatory dossiers, toxicological profiles, risk characterization, clinical assessments, and claim substantiation for various nutraceutical and well-being products. Our expertise extends to drafting risk assessment profiles for probiotics, ensuring compliance with the dossiers required for GRAS (Generally Recognized As Safe) Notification in the United States.
Probiotic Assessment Services: Evalueserve offers comprehensive support in preparing risk-benefit assessment profiles, target population profiles, and other regulatory requirements for probiotics. Our process adheres to all FDA-mandated steps for GRAS notification dossiers highlighted, including:
- Clinically Documented and Validated Health Benefits: We ensure robust clinical documentation and validation support all health benefits claimed.
- Pathogenicity and Virulence: We assess probiotic strains’ potential pathogenicity and virulence to ensure safety.
- Production of Biogenic Amines or Other Potentially Toxic Metabolites: We evaluate the output of any biogenic amines or other potentially toxic metabolites to mitigate risks.
With Evalueserve’s hands-on experience and meticulous approach, we have helped many clients across geographies navigate the complex regulatory landscape, ensuring their probiotic products meet all necessary safety and efficacy standards in the past and continuing with this pursuit. The detailed steps are highlighted in the Figure 1, given below:
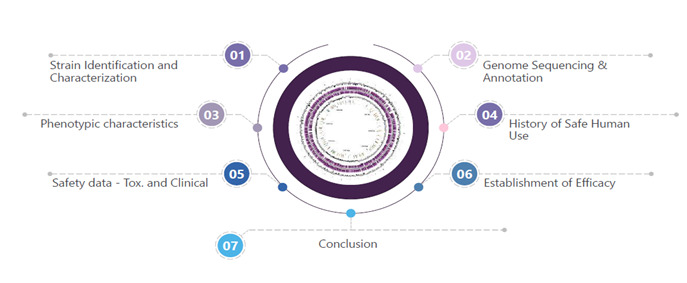
Conclusion
Including probiotics in antibiotic therapy represents a promising frontier in healthcare. It aims to balance the benefits of antibiotics with maintaining a healthy gut microbiota. This trend offers businesses in the probiotic assessment sector a lucrative opportunity to innovate, educate, and lead the market in developing safe, effective, personalized probiotic solutions. By tapping into this growing demand, businesses can enhance patient health outcomes and establish a strong foothold in a burgeoning industry.
Talk to One of Our Experts
Get in touch today to find out about how Evalueserve can help you improve your processes, making you better, faster and more efficient.


