Research and development in MedTech companies accounts for approximately 6 to 7 percent of their revenues. This high cost stems from an extensive and costly process—identifying viable innovations, conducting trials, securing FDA approval, and ensuring scalable manufacturability. According to Deloitte's analysis in The Digital Era in the MedTech Industry, R&D spending has historically remained stable—between 6.1% and 6.9% of revenues from 2009 to 2022—despite increasing downward pressure on profit margins.
However, the MedTech industry is undergoing a profound transformation. Digital technologies are reshaping how companies design, test, and launch medical devices, introducing unprecedented efficiency, speed, and precision. Key technologies such as rapid prototyping, virtual reality (VR), digital twins, and artificial intelligence (AI) are at the forefront of this revolution, enabling limitless iteration and refinement of medical device concepts. In this blog, we explore how these innovations are redefining device design and testing—ultimately contributing to safer, more innovative, and more cost-effective healthcare solutions.
This blog continues the conversation by exploring how MedTech companies embrace these technologies for faster, smarter, and safer product development. Building on the foundation laid by Rani Holani's blog on MedTech digitalization, this post explores how companies are transforming product development through AI, digital twins, and 3D prototyping.
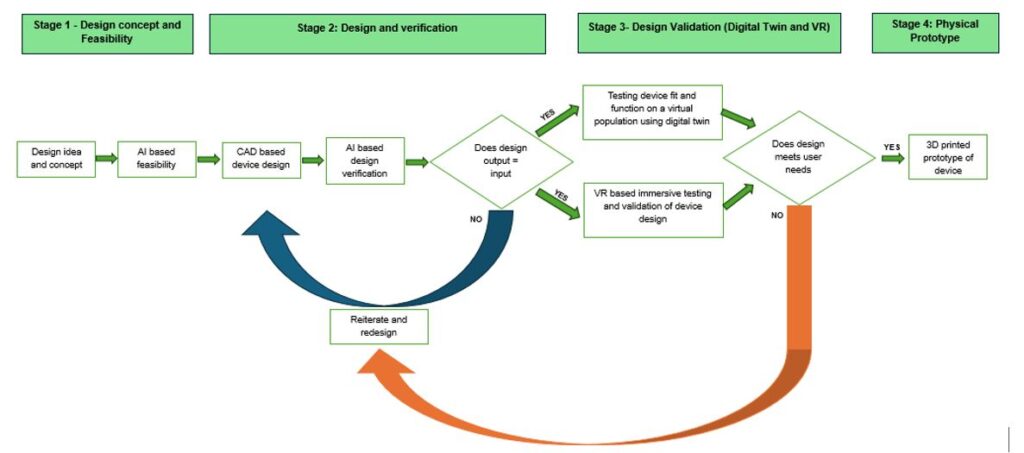
Stage 1: Smart Design Concept and Feasibility
The digital device design process begins with an idea grounded in thorough market research to uncover unmet user needs. This approach includes collecting inputs from key stakeholders such as doctors, nurses, technicians, patients, and hospitals to shape concepts that provide real clinical utility.
The idea’s overall feasibility is assessed from technical, financial, and risk perspectives:
- Technical Feasibility: AI algorithms can analyze vast datasets to predict the performance and compatibility of proposed concepts with existing technologies.
- Financial Feasibility: AI models forecast market trends, pricing strategies, and manufacturing costs, offering more accurate ROI projections.
- Risk Assessment: AI identifies potential clinical and development risks by analyzing historical data, enabling proactive mitigation strategies.
Integrating AI into these assessments ensures a robust, data-driven approach and increases the likelihood of successful product development.
Stage 2: Smart Design and Verification
After finalizing the initial idea, stakeholders provide detailed design inputs regarding desired features, functionality, and performance. Regional regulatory documents, including ISO standards and FDA guidelines, are also reviewed to ensure biocompatibility, electrical safety, and usability compliance.
Designers then use computer-aided design (CAD) software such as SolidWorks to create a digital device version. This stage allows for multiple iterations to optimize fit, functionality, and aesthetics without the delays of traditional manufacturing processes.
AI-based tools further verify whether the design meets stakeholder requirements and regulatory standards. If it does, it moves to validation. If not, iterative improvements are made efficiently within the digital environment.
Stage 3: Design Validation (Digital Twin and VR)
Once verified, the CAD model is integrated with a digital twin representing a virtual population of patients. This stage allows simulations of how the device will interact with the human body, including anatomical and physiological tests. Digital twins represent average and variant anatomies, ensuring broader patient coverage and enhanced clinical relevance.
In contrast, VR provides an immersive 3D view of the medical device. Teams can explore spatial relationships, ergonomics, and design functionality in a simulated environment. VR bridges the gap between bench testing and animal trials and enables collaborative design reviews with global stakeholders interacting in real-time.
Stage 4: Physical Prototype
Once the digital model is validated, 3D printing creates physical prototypes. This stage allows for developing complex geometries and custom designs that were previously unfeasible or cost-prohibitive.
Multiple prototypes can be produced and compared to identify the best-performing version for further development. Companies like Stratasys and Materialise successfully use this approach for products such as dental aligners, orthopedic implants, and point-of-care diagnostic devices.
Conclusion: A Digital Future for MedTech
The digital transformation of the MedTech industry is not merely a trend but a strategic imperative. AI-driven feasibility studies, CAD design, VR simulations, digital twins, and rapid prototyping collectively form a robust framework that enhances innovation while lowering risk.
Forward-Looking Insight: In the next five years, we anticipate digital twin validation becoming a formal component of regulatory submissions. Companies that embrace these tools today will be well-positioned to lead tomorrow.
Talk to One of Our Experts
Get in touch today to find out about how Evalueserve can help you improve your processes, making you better, faster and more efficient.


