The global push toward sustainability transforms how consumer products are developed, assessed, and brought to market. New environmental directives—"Green Laws"—are raising the bar for safety, transparency, and accountability across the chemical and consumer goods sectors. This transformation is not just regulatory—it's cultural. Consumers are more informed, regulators are more proactive, and businesses are more exposed to risk if they fail to adapt. In this evolving context, rethinking chemical safety isn’t just a compliance exercise—it’s a strategic imperative.
Developing safe and sustainable products isn't just a compliance exercise in this evolving context. It's a strategic imperative. But the path forward is fraught with complexity. Chemical companies and consumer brands must navigate a patchwork of regional regulations, respond to rising scrutiny of product ingredients, and do so while staying ahead in innovation and Intellectual Property (IP) protection.
At Evalueserve IP and R&D, we help companies rise to this challenge through an integrated approach that combines expertise in IP, R&D, toxicology, and regulatory affairs. In this blog, I explore the key challenges and opportunities in developing safer consumer products and share how our Chemical Safety Group supports innovation with confidence.
What Makes Product Safety So Complex Today?
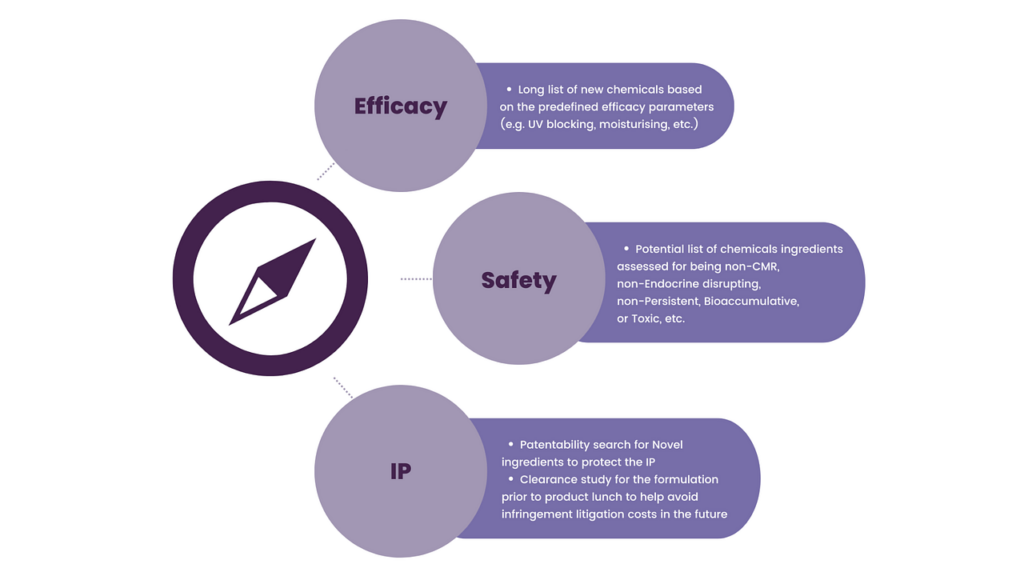
Product safety is no longer a checkbox—it’s an ongoing commitment that spans the entire product lifecycle. Companies face mounting challenges, including:
- Increased consumer awareness and demand for transparency
Today’s shoppers are questioning what’s in their products and what those ingredients mean for their health and the environment.
- Ever-evolving global regulations
Governments are tightening controls, but the rules vary widely across jurisdictions, from Europe’s REACH legislation to localized bans in states like Hawaii.
- The difficulty of harmonizing global safety standards
A chemical deemed safe in one market may be restricted or banned in another, complicating global product development strategies.
- Ingredient innovation pressures
Replacing controversial or outdated substances requires identifying new ingredients that deliver equivalent efficacy with less environmental or health impact.
- Access to reliable toxicological and environmental data
Many emerging compounds lack comprehensive data, requiring advanced methods to assess safety risks.
- Strategic IP protection and competitive pressure
Innovating new chemical formulations or ingredients means protecting them early and effectively across markets—especially when patenting chemical compounds or conducting landscape searches in multiple languages.
A Closer Look: The Case of Octinoxate (OMC)
One example that illustrates this challenge is Octinoxate (OMC), an organic compound historically used as a UV filter in sunscreens. Also known as ethylhexyl methoxycinnamate, this chemical has been a mainstay in personal care products for decades. However, its safety and environmental profile are under growing scrutiny.
Global Regulatory Backlash
- EU: Caps OMC in cosmetics at 10%
- US (FDA): Allows up to 7.5% in sunscreens.
- Australia (NICNAS): Permits concentrations under 10%
- Thailand and parts of the US (e.g., Hawaii) have banned its use in sunscreens due to coral reef damage.
OMC is now listed on the EU Watchlist and under evaluation as a potential endocrine disruptor. It has also been found in aquatic environments globally, raising alarms about bioaccumulation and coral bleaching, especially in marine tourism hubs.
Is OMC a Human Health Risk?
The debate is ongoing. Non-profit scientific bodies such as the Haereticus Environmental Laboratory cite animal studies that point to reproductive and hormonal disruptions. However, these conclusions are based mainly on in vitro tests or high-dose animal studies.
Australian authorities (NICNAS) have concluded that the ingredient poses no unreasonable health risk to the public at current exposure levels. Similarly, a review by Suh et al. (2020) found no conclusive evidence of thyroid or reproductive hormone disruption in humans.
Nonetheless, regulatory bodies such as the Scientific Committee on Consumer Safety (SCCS) in Europe are awaiting more data, signaling that the final verdict is still to come.
Environmental Impact: A More Urgent Concern
While human toxicity remains under investigation, environmental risks are more clearly documented. Studies confirm that OMC contributes to coral reef damage by interfering with the symbiotic organisms that keep corals healthy. This impact has led to increasing global momentum to phase out the ingredient, especially in regions dependent on marine ecosystems.
How Can Companies Find Safer Ingredients?
Replacing high-risk ingredients like OMC requires more than simply finding a chemical substitute. It demands a proactive safety strategy that combines:
- Screening for safer functional analogs
Ingredients must meet both safety and efficacy criteria.
- Utilizing alternative data methods
When traditional safety data are missing, in silico models, in vitro studies, and computational toxicology can fill the gaps.
- Conducting longitudinal studies
Observational studies and post-market surveillance can offer real-world insights.
- Understanding regional regulations in advance
Businesses must align R&D decisions with the future direction of local and global regulations.
- Ensuring robust IP protection
Companies must secure the freedom to operate and protect investments through strong patent strategies.
Evalueserve's Role: Supporting Safer Innovation
At Evalueserve, we help companies take a structured, evidence-driven approach to safer product development. Our services include:
- Ingredient Landscaping: We scout for novel chemical ingredients that offer safety, efficacy, and commercial viability.
- Toxicological Data Screening: Our Chemical Safety team reviews and analyzes safety data from various sources, ensuring data quality and completeness.
- Regulatory Assessment: We interpret and track evolving legislation globally to help companies stay ahead.
- Advanced IP Searches: We conduct multilingual patent searches to support early-stage R&D and freedom-to-operate assessments.
- Methodology Consulting: Our experts advise integrating next-gen testing tools, from in silico models to alternative risk assessments.
We aim to help clients make smart, safe decisions from the outset—reducing risk, speeding time to market, and building long-term trust with consumers and regulators alike.
Final Thoughts
The future of consumer products is rooted in safety, sustainability, and scientific credibility. Navigating the increasingly complex regulatory and innovation landscape is not optional—it's essential. Companies can develop products that meet market demand and regulatory scrutiny by leveraging cross-disciplinary expertise and next-generation tools.
At Evalueserve, we're proud to help companies lead the way in safe and sustainable innovation.
Talk to One of Our Experts
Do you need help assessing your ingredients or building a safer innovation pipeline? Contact our team to explore how we can support your R&D and regulatory goals.


