In early 2024, the European Chemicals Agency (ECHA) launched ECHA CHEM, marking a pivotal shift in how chemical safety data is managed, accessed, and regulated. This transformation isn't merely a digital upgrade—it's a strategic overhaul built on automation, artificial intelligence (AI), real-time data access, and a renewed commitment to transparency and compliance.
As regulatory demands grow and the complexity of chemicals increases, ECHA CHEM aims to modernize chemical safety management across Europe, aligning seamlessly with evolving REACH and CLP regulations, sustainability goals, and the Globally Harmonized System (GHS).
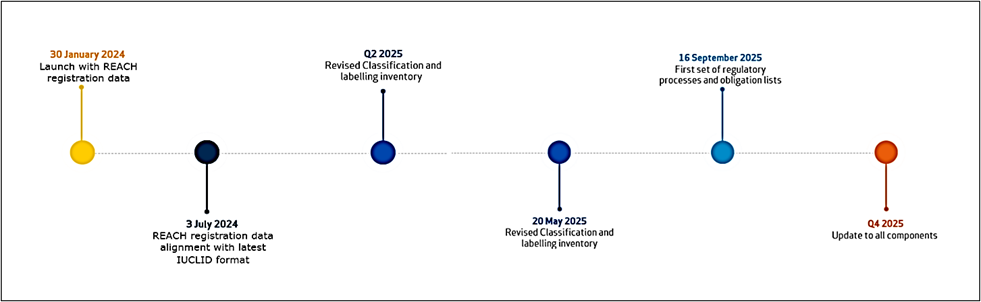
Timeline of ECHA CHEM Implementation
To fully understand the significance of ECHA CHEM, it’s essential to examine how the system has evolved. The following timeline outlines key system milestones and updates from its initial launch through planned improvements later in 2025.
Driving Forces Behind the Transition
With the timeline established, let’s explore the key drivers behind this digital transformation. ECHA CHEM was not built in a vacuum—it addresses urgent operational, regulatory, and technological needs across the industry.
1. Growing Data Volume and Complexity
The number and complexity of chemicals requiring registration are skyrocketing. Companies must submit detailed data on physical, environmental, and toxicological properties. The old ECHA system struggled with slow processing and data overload, often causing bottlenecks and timeouts.
New vs. Old Registration Process:
| ECHA (Old) | ECHA CHEM (New) |
| Manual verification | AI-driven validation |
| 5–7 days processing | Completed in 1–2 days |
| Queued submissions | Real-time handling |
While data volume was a significant challenge, evolving compliance requirements added another layer of urgency for systemic change.
2. Evolution of Regulatory Requirements
REACH and CLP updates—such as polymer and nanomaterial regulations—have added complexity. ECHA CHEM is built to dynamically align with these changes and the globally harmonized GHS framework, supporting consistent classification and registration across regions.
These regulatory shifts were only part of the equation—technological modernization was equally necessary to support this transformation.
3. Digital Transformation Demands
ECHA CHEM replaces manual processes with automation and AI, improving:
- Efficiency (faster processing)
- Accuracy (reduced human error)
- Scalability (handles big data)
- Cost-effectiveness (fewer manual resources)
AI Highlights:
- Predictive toxicology
- Risk prioritization
- Pattern recognition for emerging hazards
It also enables real-time updates, better monitoring, and transparency for all users.
With this digital foundation in place, ECHA CHEM introduced a suite of advanced functionalities that redefine user experience and compliance efficiency.
Key Functional Enhancements in ECHA CHEM
|
Category |
New Features |
|
Search |
Multi-parameter filtering, real-time results, better categorization |
|
Data Management |
Lifecycle tracking, regulatory cross-integration, dossier completeness |
|
User Interface |
Mobile-ready, customizable dashboards, data visualizations |
|
Compliance |
Visual alerts, public consultation tools, automated checks |
These innovations have continued to evolve through 2025 as the system adapts to new regulatory demands and feedback from its users.
Mid-2025 System Updates & Milestones
ECHA CHEM’s progress continues through 2025 with new releases and improvements that reflect its evolving role in the regulatory ecosystem.
ECHA CHEM continues to evolve:
- IUCLID 6 update: Supports new REACH & EU directives
- Registration Restart (Planned for Sep 2025): Adjusted for system integration
- Candidate List: 3 new substances added, total now 250 SVHCs
- SME Support (July 2025): New helpdesk tools, UI simplification, and pre-submission guidance
These upgrades demonstrate ECHA’s responsiveness to industry needs. But what do they mean for specific stakeholders?
Impact on Stakeholders
For Industry
- Streamlined submission processes
- Clearer guidance on requirements
- Reduced compliance burden
- Better access to regulatory updates
- Enhanced data reporting capabilities
For Regulators
Automation, AI, ML, and real-time data processing enable:
- More efficient evaluation processes
- Better substance tracking
- Improved enforcement capabilities
- Enhanced decision-making tools
- Streamlined communication channels
For Researchers
- Better access to chemical safety data
- Advanced analytical tools
- Comprehensive substance information
- Improved data export capabilities
- Enhanced research collaboration options
Sustainability and Environmental Focus
- Promotion of Safer Chemicals: Support for the development of green alternatives.
- Enhanced Environmental Impact Assessments: Better tracking of persistent pollutants.
- Alignment with EU Sustainability Goals: Integrated support for environmental policies.
As promising as these results are, ECHA CHEM is still evolving—poised to offer even greater value in the years ahead.
Future Outlook
ECHA CHEM is a transformative step in chemical regulation, but its potential is far from fully realized. Future developments may include:
- Continuous improvements and adaptations
- Integration of emerging technologies
- Enhanced global harmonization
- Better support for sustainable chemistry
- Improved stakeholder engagement
Conclusion
The transition from ECHA to ECHA CHEM marks a new era in chemical regulation—one where automation, AI, and real-time processing power create a safer, more transparent, and more efficient ecosystem for chemical data and regulatory compliance.
As Europe prepares to resume complete REACH processing in September 2025, staying updated on ECHA CHEM developments is critical for companies, researchers, and regulators alike.
Need Support with ECHA CHEM? Meet Rasayan.
At Evalueserve IP and R&D, our Chemical Safety & Regulatory Affairs (CSRA) team developed Rasayan—a dedicated tool to:
- Sanitize ECHA/ECHA CHEM data
- Summarize complex dossiers into usable insights
- Automate tracking and compliance mapping
Whether you’re managing substance registration or monitoring regulatory changes, Rasayan can reduce manual effort and improve accuracy.
Contact our team to learn how Rasayan fits your workflow.
Talk to One of Our Experts
Get in touch today to find out about how Evalueserve can help you improve your processes, making you better, faster and more efficient.



