Have you ever considered the hidden risks that could jeopardize the longevity of your patent portfolio? Recent rulings, particularly the Cellect decision, have uncovered vulnerabilities in patent families where Patent Term Adjustments (PTA) were awarded. As patent owners, practitioners, and legal professionals, it’s essential to ask: Could your portfolio be at risk for invalidation due to Obvious-Type Double Patenting (ODP) challenges? With significant implications on PTA terms, these rulings demand a strategic rethink in managing and defending patent portfolios.
Who and Why Should You Read This?
The recent Cellect ruling has highlighted significant invalidation risks for patent families with PTA extensions. Specifically, the ruling highlights how patents awarded PTA but also have ODP risks—i.e., patents that are not distinct within the family—could now be subject to ODP attacks, making them vulnerable to litigation at later stages.
Why: This case highlighted the invalidation risk associated with patent families where the PTA term was awarded to members with ODP risk, i.e., members of a patent family claiming subject matter that might be considered patentably indistinct. Due to this ruling, these members could now be vulnerable to later-stage litigation attacks using ODP PTA challenges.
This article talks about the Cellect ruling, which includes discussion on:
Who: If you are a Patent owner, practitioner, or legal professional involved in patent litigation and portfolio management, you should read this to understand the impact.
Key Takeaways
The Cellect ruling introduces a critical new risk factor for patent portfolios. It underscores the potential for invalidation of patents with statutory PTA due to ODP, where earlier-expiring family members with no or shorter PTA terms are used as the basis for these attacks. This ruling stresses the need for caution and proactive measures in managing patent families. Let’s explore these impacts in more detail:
- In Cellect ruling: The Federal Circuit’s decision has introduced a potential risk factor. It ruled that patents with statutory PTA could be invalidated due to ODP by earlier-expiring patents within the same family with no or shorter PTAs. This ruling underscores the need for caution and proactive measures in managing patent portfolios.
- In the Allergan USA v. MSN Lab ruling, It was clarified that “a first-filed, first-issued, later-expiring claim” parent patent cannot be invalidated using ODP by a child patent having “a later-filed, later-issued, earlier-expiring reference claim having a common priority date.”
- It is interesting to note that an amicus brief filed by the New York Intellectual Property Law Association (NYIPLA) in May 2024 cited data from the USPTO, stating that half of all patents issued by the USPTO receive PTA, averaging 299 days added to patent exclusivity for USPTO delays. The NYIPLA’s involvement in this case underscores the significance of the Cellect ruling and its potential impact on patent term adjustments.
- The first-filed, first-issued patent within a patent family often receives the most PTA and cannot be invalidated for ODP over subsequent patents in the same family. Pursuing this application’s most critical subject matter would set the maximum exclusivity period for the claimed subject matter and any patentably indistinct variants.
- Practitioners should consider PTA and terminal disclaimer strategies during filing and litigation.
- Monitoring both one’s own and competitors’ patent portfolios for PTA-related ODP vulnerabilities is now crucial due to the increased probability of this type of attack. Filing the Terminal disclaimer could severely impact the entire family, as in the Cellect ruling.
The August 2024 ruling in Allergan USA v. MSN Laboratories brought more clarity to the earlier 2023 Cellect ruling, indicating that this is an evolving situation that will be further clarified through case proceedings on a case-by-case basis. Though the ruling is still evolving, it is essential to proactively keep track of any potential issues with your patent portfolio and take any preventive measures earlier rather than later upon detection of any problem, as not doing this may leave you open to ODP PTA attacks, which in some cases can be fatal, like in the case of the Cellect patent family in the Cellect ruling.
Case Background in Detail
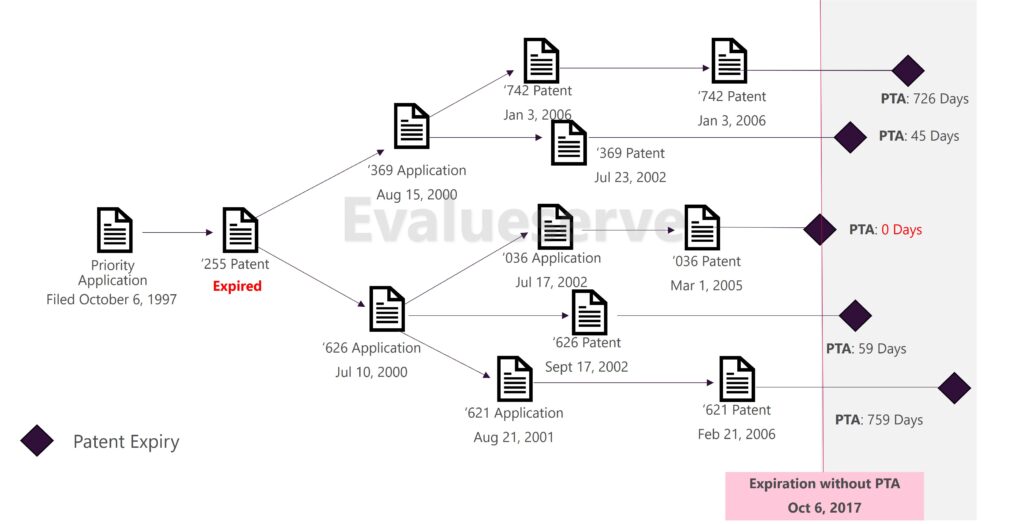
On August 28, 2023, the U.S. Court of Appeals for the Federal Circuit issued a ruling in the case of In re Cellect LLC, 81 F.4th 1216, 1228–29 (Fed. Cir. 2023), involving a dispute between Samsung and Cellect regarding a patent family of five Cellect patents related to image sensors. Under normal circumstances, these patents would have expired at the same time. However, four were granted extended terms due to Patent Term Adjustment (PTA). The last patent filed was the only one that did not receive a PTA extension.
On August 28, 2023, the Federal Circuit ruled that patents with statutory Patent Term Adjustments (PTA) could be invalidated due to obviousness-type double patenting (ODP) by earlier-expiring patents within the same family with no or shorter PTAs. This decision upheld the Patent Office’s Patent Trial and Appeal Board (PTAB) ruling. The court clarified that when patents within a family have different expiration dates solely due to PTA, earlier-expiring family members can be used as the basis for an ODP validity attack on later-expiring family members.
The Federal Circuit provided further clarification in Allergan USA v. MSN Laboratories Pte. Ltd. (2024), clarifying that although Cellect established a rule that courts must assess ODP based on expiration dates after PTA has been added, it explained that a parent patent receiving PTA causing it to expire after a child patent is not invalid under ODP. The court highlighted that the objective of the ODP doctrine is to stop patentees from securing a second patent for an invention that is not significantly different from the first, thereby preventing an extension of the original patent’s lifespan.
Note: PTA addresses administrative delays caused by the United States Patent and Trademark Office (USPTO) during the examination. PTE is awarded to compensate for delays due to the regulatory approval (FDA) process.
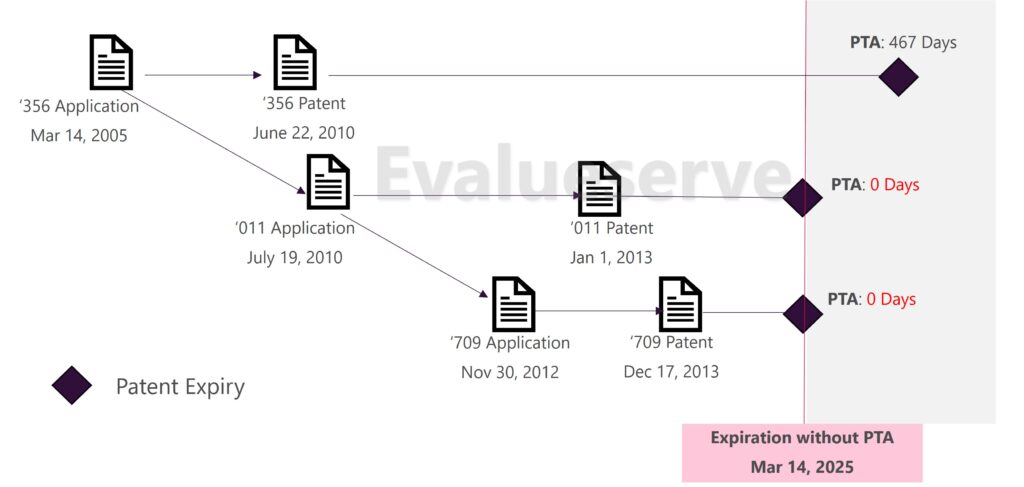
It is important to note that without PTA and PTE, all patents in the same family would have expired with the parent application. Different PTA or PTE terms awarded within a family mainly cause different members within the same family to have artificially prolonged lives, resulting in potentially different expiry dates for each member of the family.
Conclusion: Impact & Precaution
The implications of the Cellect ruling are significant for patent owners and practitioners:
- PTA and ODP Analysis: The Federal Circuit’s ruling in the case of In re Cellect, LLC, has significant implications. When members of a patent family have different expiration dates due to PTAs, the earlier-expiring patents can invalidate the later-expiring ones based on ODP. The Federal Circuit concluded that the expiration date used for an ODP analysis where a patent has received a PTA is after the PTA has been added. This means that patents with PTA are vulnerable to ODP attacks.
- What about PTE? The Federal Circuit confirmed that differences in expiration dates caused by PTE do not trigger ODP as PTE is applied after ODP analysis. In contrast, PTA is applied to the patent term before the ODP analysis. This distinction is crucial because it means that while PTA can be voided during ODP analysis, PTE remains unaffected.
- Terminal Disclaimer: Without a terminal disclaimer, a related patent that has received more PTA terms can be invalidated by another earlier-expiring family member claiming subject matter that might be considered patentably indistinct via ODP attack.
- Patent Strategy:
- Defensive: File terminal disclaimers before the earlier-expiring patent expires to cure any claim defects due to ODP. Monitor patent families closely to ensure compliance with the new ruling. Alternatively, take the risk of filing the terminal disclaimer later, preserving the additional term but putting the patents at risk if the earlier expiring patent expires.
- Offensive: Patent owners should assess their competitors’ patent portfolios to look for patents with PTA that are now potential targets for an ODP challenge under Cellect. Use the ruling to invalidate competitors’ later-expiring patents within the patent family strategically. This is useful during patent litigations, especially when you are faced with attacks from multiple patents with similar claims.
- Prosecution and Litigation: The ruling affects the strategic decision of when to disclaim a patent challenged on ODP grounds terminally. Patent owners should work with experienced litigation counsel to navigate these complexities.
Moving forward
The evolving nature of PTA and ODP issues requires expertise and foresight. At Evalueserve IP and R&D, we offer comprehensive support to help patent owners navigate these legal complexities. Our team can assist in protecting your patent portfolios from potential ODP attacks and help identify weaknesses in competitors’ patents. Don’t wait for these risks to materialize—contact us to safeguard your intellectual property and make informed, strategic decisions in this changing landscape.
Disclaimer: This article does not constitute legal advice and is only for general information. Always consult your legal counsel for specific legal guidance.
Talk to One of Our Experts
Get in touch today to find out about how Evalueserve can help you improve your processes, making you better, faster and more efficient.